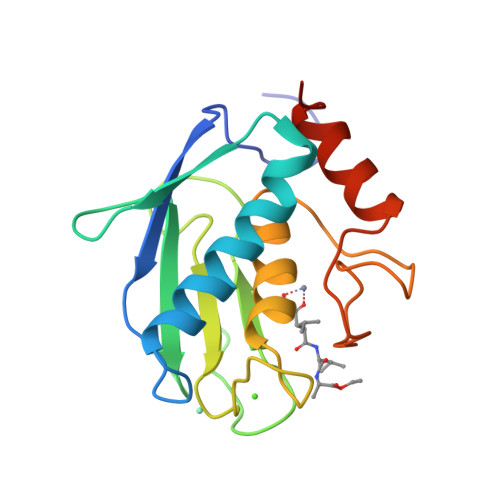
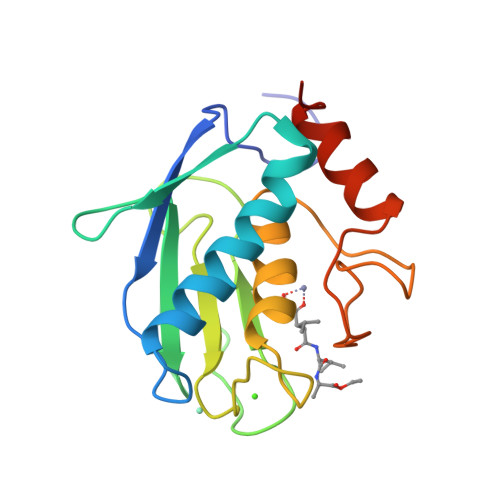
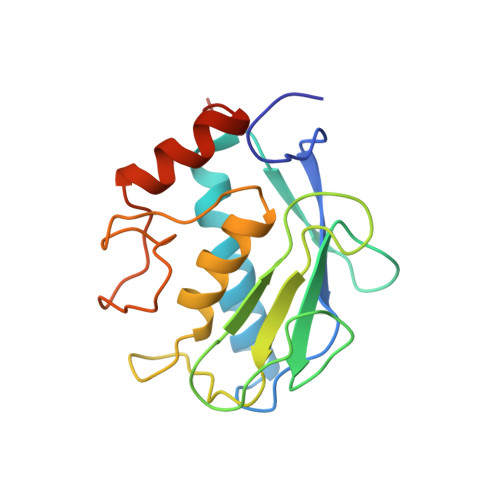
Structure of the catalytic domain of human fibroblast collagenase complexed with an inhibitor.
Borkakoti, N., Winkler, F.K., Williams, D.H., D'Arcy, A., Broadhurst, M.J., Brown, P.A., Johnson, W.H., Murray, E.J.(1994) Nat Struct Biol 1: 106-110
- PubMed: 7656013
- DOI: https://doi.org/10.1038/nsb0294-106
- Primary Citation of Related Structures:
2TCL - PubMed Abstract:
In rheumatoid and osteoarthritis, degradation of articular cartilage is mediated by the matrix metalloproteinases collagenase, stromelysin and gelatinase. The key event in this process is the cleavage of triple helical collagen by collagenase. We have determined the crystal structure of the catalytic domain of human recombinant fibroblast collagenase complexed with a synthetic inhibitor at 2.2 A resolution. The protein fold is similar to the amino termini of the zinc endopeptidases astacin thermolysin and elastase despite a lack of primary sequence homology. The conformation of the bound inhibitor provides a molecular basis for the design of inhibitors of collagenase and other matrix metalloproteinases. Such inhibitors should be useful in the treatment of a variety of diseases including arthritis and cancer.
Organizational Affiliation:
Roche Products Ltd, Garden City, Hertfordshire, UK.